
The pelvis plays the critical role of supporting the spinal column and protecting abdominal organs. Ordinarily is it an elegant, butterfly-shaped grouping of bones and ligaments which includes the sacrum (base of the spine); however, an internet search of pelvis fractures delivers jarring images of long and large surgical pins, screws and plates being used (in and outside the body) to fixate the pelvis for healing. These are accompanied by similarly unsettling phrases like: almost always painful; difficulty moving or walking; high-energy trauma; loss of nerve function. Together, these paint an accurate portrayal of the seriousness of pelvic fractures. For many patients, these potentially life-threatening injuries may include organ damage or other related injuries, and require emergency medical care and lengthy physical therapy and rehabilitation.
CurvaFix, a UBC spin-off company, launched the CurvaFix® IM Implant in 2021, with the hope of radically changing these outcomes. This medical implant is the first real innovation in pelvic-trauma technology in 30 years and is the only pelvic fracture fixation device capable of being implanted inside the bone and following its curve. With the CurvaFix® IM Implant, pelvic fracture fixation is minimally invasive, requiring just one small incision and drill hole per implant. And, the device has an ultra-sleek interlocking design that is highly efficient and just plain looks cool.
From ideation to prototype development
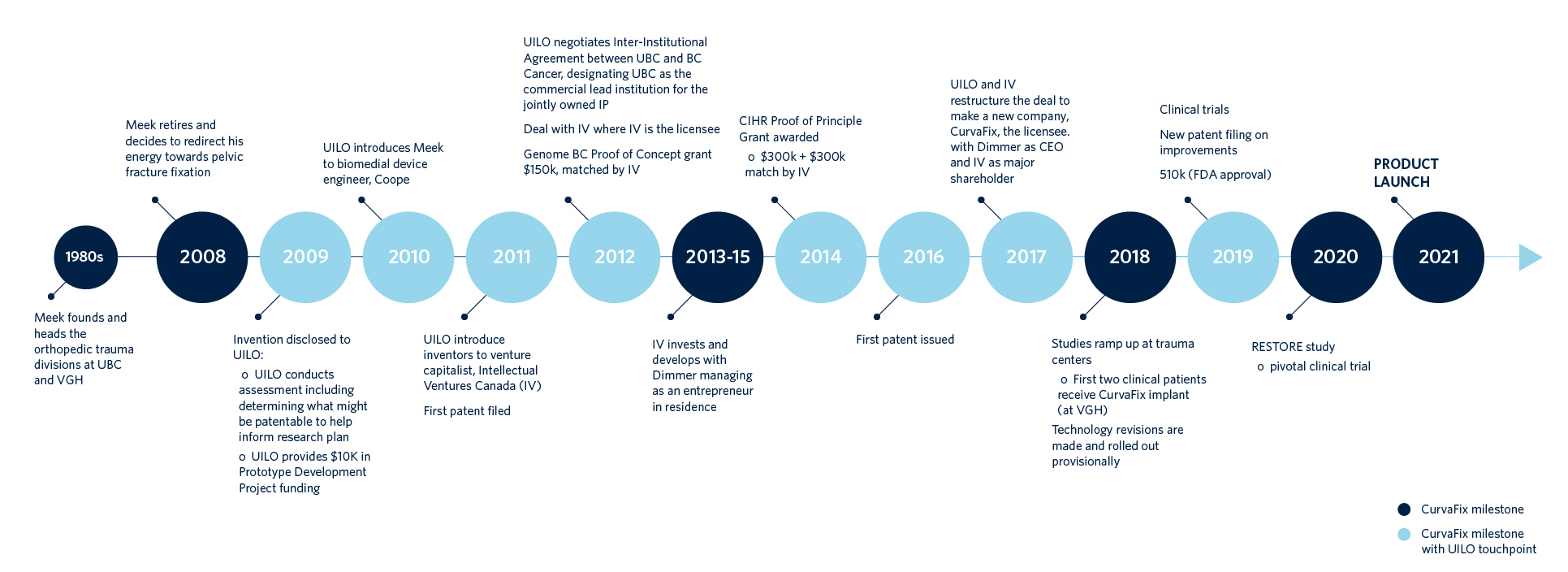
The CurvaFix® IM Implant is the brainchild of Dr. Robert Meek (BSc ’64, MD ’68), a clinical professor emeritus in UBC’s Faculty of Medicine and a retired orthopedic trauma surgeon. “The main issue is that devices used currently were never developed for the pelvis,” he says. “They were modifications of techniques that had been developed for other surgeries, but the pelvis is unique in that its bones are curved.” Long vexed by the inadequacy of existing pelvic fracture fixation techniques, upon retiring as a surgeon, Dr. Meek was finally able to devote his attention to finding a better solution. “The pelvis holds almost all your body weight when you walk or even sit, so I thought we could put something curved in there and sort of fill up the whole inside of the bone, but it would need to be both strong and rigid.” In 2008, biomedical engineer and orthopaedic biomechanics expert Dr. Thomas Oxland (UBC Professor, Orthopaedics and Mechanical Engineering; Associate Head, Research, Department of Orthopaedics at Vancouver General Hospital (VGH); and Associate Director, the ICORD Research Centre), advised Dr. Meek that his idea was viable and worth pursuing. But also, valuable, and that in order to protect the intellectual property (IP) of his invention, he should establish a relationship with UBC’s University-Industry Liaison Office (UILO).
“I could have gone private and established a business myself: hired a lawyer, negotiated and managed all the different things,” says Dr. Meek. But, he wasn’t interested in this project to explore his entrepreneurial skillset; what Dr. Meek wanted was to get this product out to market as efficiently as possible in order to start improving health outcomes for pelvic-fracture patients.
Dr. Meek established connection with the UILO in 2009. Through Brad Wheeler, its technology transfer manager who specializes in working with UBC’s Affiliated Hospitals, he disclosed his idea and brought it into development via a $10,000 UILO Prototype Development Project funding grant. In 2010, Wheeler connected Dr. Meek with Dr. Robin Coope (MASc ENPH ’96, PhD PHYS ’05), at the BC Cancer Agency’s Genome Science Centre, a professional engineer who specializes in biomedical hardware, instrumentation and device development. “I designed a first rendition myself” says Dr. Meek, “but it was very, very primitive.” Together they began to refine the implant’s design.
Developing and raising CurvaFix Inc.
After a year of successful early development, Wheeler then connected with Intellectual Ventures Canada (IV), which had been acquiring Canadian intellectual property in order to develop and re-license it. As a favour to IV, medical device entrepreneur Steve Dimmer (now, President and CEO of CurvaFix) flew to Vancouver to see if the device was worth their investment.
“First impressions were good” says Dimmer. “The inventors seemed honest and ethical and while I didn’t have a background in orthopedics at the time, the technology and methodology seemed like it could have potential, and I was excited for the possibility to make a difference for patients … They knew their problem space very well, but what they didn’t know was the market: How big is it? Could it go worldwide? What would pricing have to look like? What does market access look like? And, technology and intellectual property were still early. Much work remained to de-risk the implant concept and develop it for clinical use.” As a result of Dimmer’s feedback IV decided to license the patent rights from UBC. With IV as the Licensee the project became eligible for industry matched grant funding from both Genome BC’s Proof of Concept grant and CIHR’s Proof of Principal Program II grant. These grants, plus significant direct funding from IV to UBC, accelerated the prototyping efforts of Drs. Coope and Meek. This work was conducted at the Centre for Hip Health and Mobility (CHHM) at VGH, which is part of the Vancouver Coastal Health Research Institute (VCHRI). At this point IV made the decision to restructure the deal into a spin-off model, with Dimmer leading the venture-building aspects. Working closely with the UILO on the restructuring, and with Dimmer at the helm, CurvaFix Inc. spun out of UBC in 2017.
“University-industry liaison offices are frequently inflexible and difficult to navigate” says Dimmer, “but Brad and the UBC UILO were flexible on the things I needed them to be flexible on; he’s experienced, and a very good listener. The support we’ve received through them has really allowed us to be able to focus on the work of getting the product to market. Dr. Meek is still deeply involved as chairman of our Surgeon Advisory Board and is an active team member, focusing on the medical aspects of the technology. CurvaFix is now a medtec start-up company which I am leading as CEO.” The connection to UBC and VCHRI has also continued, notably with device prototyping at BC Cancer, as well as cadaver studies and mechanical bench testing at the CHHM’s anatomy lab. An important lesson in moving the innovation toward impact says Dr. Coope, is the value of the connections facilitated through the UILO, especially that to Steve Dimmer. “Steve is incredible at finding the right people to make the next parts of this thing happen properly: whether it’s FDA approval; marketing; finding our incredible sales team; securing investors… all these things are part of a CEO skillset.”
CurvaFix enters the OR
In March 2019, CurvaFix received FDA clearance — a legal requirement to be able to market and sell medical devices in the United States — followed by Health Canada Approval and its first two in-human studies at VGH. In 2020, further equity financing was raised and an initial thirty-patient clinical study, called RESTORE, previewed for the CurvaFix team just how impactful their technology could become. Due to its oval shape, the pelvis almost always breaks in at least two places (like breaking a LIFE SAVER candy), and requires fixation for each breaking point. Surgeons in the RESTORE study trained on the theory and technique of using the CurvaFix® IM Implant and after a little practice, most could implant the device in about 20 minutes. While the surgery time is approximately the same as existing pelvic-fixation techniques, the advantage is that instead of patients being largely immobilized and/or in significant pain, the CurvaFix® IM Implant’s design achieves strong fixation that allows them to engage in weight-bearing activities (like sitting up, or using a walker) with little to no pain as early as the day after surgery.
The magic of the CurvaFix® IM Implant is in its design. The stainless steel implant is constructed from interlocking segments that resemble a snake made of puzzle pieces, and the physics are similar to that of how a suspension bridge works. Inserted through a small incision, and using standard fluoroscopy, its patented technology uses tension and transfer of force to create stability. A flexible drill bit creates a curved tunnel through the fracture, then guidewires are inserted for the rod implant. Once the implant is in place the surgeon rotates the lock, making the curved device rigid by holding the internal cables at fixed lengths. And that’s it; the pelvis is solid and secure! “If you have a broken bone, it really only hurts when it’s wiggled,” explains Meek. “If you can make it immobile, it really doesn’t hurt. Maybe a patient has pain from associated soft tissue, but compared to bone breakage, it is quite tolerable. What the CurvaFix implant is doing is stabilizing the fractures so they don’t move and hurt. We can get people up and moving around quite quickly!” As a former orthopedic surgeon, he is very aware of the impact of pain on overall health and patient outcomes. “Being on strong narcotics and lying immobilized in bed can cause medical complications — pneumonia; bed sores; urinary tract infections — that are very serious for multiply injured patients, and very, very serious for the elderly. Mortality is relatively high in those patients.” With a soft launch in 2021, the CurvaFix implant is now available in major trauma centres in the US.
Impact on patients: success and next steps
The company celebrated its one hundredth patient in September 2022, and is busy growing the business. “It’s a fun time,” says Dimmer. “And there’s lots of heartwarming stories of helping people who are suffering.” One of these examples is that of a 68-year-old woman whose fall in the shower resulted in a Y-shaped sacral fracture, and non-displaced fractures of the superior and inferior pubic ramus. Essentially, her pelvis was both broken and separated from her spine. A common trajectory for patients in such cases would be six or more weeks of limited-motion bedrest, often in a care facility, hoping for the injury to heal naturally. Alternatively, surgeons may have conducted a much more intensive surgery to install hardware that would reconnect her spine and pelvis. Multiple operations may have been required.
“Our surgeons installed just one CurvaFix device, across both sides of her pelvis in order to secure it to the upper sacrum and therefore to the spine. Two weeks post-op she was taking only acetaminophen for pain. In six weeks she was back to her normal life. And that’s just one of many equally impressive success stories” Says Dimmer. Dr. Meek, Dr. Coope and Dimmer and the CurvaFix team meet weekly to continue to propel their vision forward. In the short term, CurvaFix is working on getting their implant into the hands of more and more surgeons across the US. In ongoing collaboration with CHHM their device prototyping continues to advance, with a second version of the product soon-to-be-released — smaller in diameter and thus able to be implanted in smaller pelves — that will keep driving their operation and patient-outcome success. They intend to obtain approvals for use internationally, and longer term we may see this breakthrough technology evolving for all sorts of orthopedic surgical needs.
“Working with Dr. Meek and Robin has been a really fun collaboration, and we’re helping so many patients” says Dimmer. “It doesn’t get much better than that!”
This story originally appeared on the Innovation UBC website and the UBC UILO website. Click to learn more.


