By Tarini Boparai & Maneeta Janjua
Our UBC Global Orthopaedics (UBC GO) Spotlight series features in-depth conversations with a UBC Department of Orthopaedics member about their involvement in global orthopaedic initiatives.
In our next spotlight, we meet UBC Biomedical Engineering graduate, and CEO of Arbutus Medical Inc., Lawrence Buchan. Through his unwavering commitment to global orthopaedic initiatives, Lawrence has harnessed innovation to address the dire shortage of surgical tools in low-resource settings. His Vancouver-based company has enabled over 100,000 surgeries across 40 countries, all while paving the way for a future where safe surgical care knows no bounds.

LAWRENCE BUCHAN
Project/Initiative:
Uganda Sustainable Orthopaedic Trauma Program
UBC’s Engineers in Scrubs (EiS) Program
Arbutus Medical Inc.
What inspired your interest in global health/surgery/orthopaedics?
When I was a grad student with UBC Biomedical Engineering, I went through one of the first cohorts of the Engineers in Scrubs (EiS) program. Through EiS, I connected with UBC Orthopaedics’ Uganda Sustainable Trauma Orthopaedic Program (USTOP) and Drs Potter, O’Brien, and Blachut, to work on the challenge of limited access to orthopedic power tools in low resource settings. Conventional battery-powered surgical drill sets can cost tens of thousands of dollars each, and they require regular maintenance or expensive replacement components. It was eye-opening to listen to USTOP’s Ugandan partners discuss how the lack of access to surgical drills can directly limit their ability to treat patients.
Our goal was to develop a radically affordable orthopedic power tool specifically for use in low-resource settings. So, our team built a sterile drill cover device – similar to a dry bag, except with an interface for a drill chuck – that allows a low cost, nonsterile battery-powered drill to be used safely in a sterile surgical field.
As we learned about the field of global surgery, we saw how this was just one small problem contributing to a bigger issue. An estimated 5 billion people lack access to safe surgical care. USTOP’s research has shown how access to surgery can improve socioeconomic outcomes, so I’m very motivated to make a positive impact in this space, given the immense need.
What did you do?
We created a business around “DrillCover Technology” called Arbutus Medical, which today is a 12-person medical device company based in Vancouver. We’re an ISO 13485 manufacturer and have 3 different product lines on the market right now. Our products have been used in over 40 different countries enabling an estimated 100,000 surgeries since 2014. We have done work with over 10 different NGOs, including USTOP and Team Broken Earth. In the last two years we have incorporated this innovation into a procedure kit that streamlines bedside skeletal traction. We’re now seeing rapid growth in the US market, which is helping drive more impact and financial sustainability.
Where did you find the funding?
We have leveraged private funding for the business as well as a variety of grants and federal programs. Engineers in Scrubs has been an NSERC funded program, so we developed the prototype for the drill with initial funding through the program itself and a couple of small innovation awards. One source that really accelerated the initiative was Grand Challenges Canada’s Stars in Global Health program. We initially leveraged a proof-of-concept grant and subsequently we secured transition-to-scale funding. VCHRI also provided important funding through their Innovation and Translational Research awards. In 2014, we incorporated as Arbutus Medical Inc., and went on to raise private capital. We have secured funding from a mix of global health foundations (Sorenson Impact Foundation), angel investors and family offices, venture capital and health-tech innovation firms (including MEDTEQ+, Nimbus Synergies, ScaleGood) as well as industry funding programs like GenomeBC’s Industry Innovation program, NRC’s Industrial Research Assistance Program (IRAP), CanExport, and PacifiCan’s Business Scale Up & Productivity program.
One thing we’re probing and looking into is sustainability. Do you think your projects were able to achieve sustainability?
Our team has shipped enough products to enable an estimated 100,000 surgeries – we view this as lasting impact for those patients. From a business standpoint, Arbutus Medical has transitioned out of the research phase into commercialization as a private business. We will continue raising investment and are driving toward operating in the green to be truly sustainable. From a health or socioeconomic outcomes perspective, our team is focused on the idea that there are many hospitals in low-resource settings that are limited in how many patients they can treat effectively because they don’t have access to our system, or any surgical power drill. Through Arbutus Medical, we are providing access to safe tools at accessible price points, and by improving the standard of care our “theory of change” is that this will lead to more efficient care, fewer complications, better health, and socioeconomic outcomes. Today, we are focused on measuring the outputs (number of surgeries enabled and market access), and in the future we will explore long term outcomes in greater detail.
Looking back, what do you think were the key insights and main barriers that you faced?
It has been nearly a decade working on this initiative, and in the process, we have learned how critical it is to forge partnerships in the ecosystem to make any kind of sustainable change. Local partnerships are critical – groups like us must do a lot of listening to ensure we’re responding to real needs and creating solutions collaboratively. Ecosystem partnerships are critical too. For example, a drill is just one very small part of the operating room and an orthopaedic surgery practice. To treat a patient, a surgeon needs training, a long list of devices, a trained team of nurses, a functioning sterile processing department, funding for that patient, and so on.
Funding is a significant barrier for any global health initiative. Access to funding often relies on the innovator having an idea for how a solution can scale. We’ve taken the approach of creating a business to scale our solution. I’m a believer that business can be a force for positive change – that by figuring out a scalable, sustainable economic model, we can maximize our positive impact.
Are you working on any projects right now? What are your future plans/implications?
What my team and I have done is an example of frugal innovation and the idea that tough constraints can produce great design. Our goal is to enable 5 million surgeries by 2030, and we’d like to see a world where every patient in need has access to safe surgical care. We plan to develop multiple products, continue to build on our partnerships, and demonstrate to the industry that you can build a great business focusing on underserved markets – and that this can have a significant positive impact.
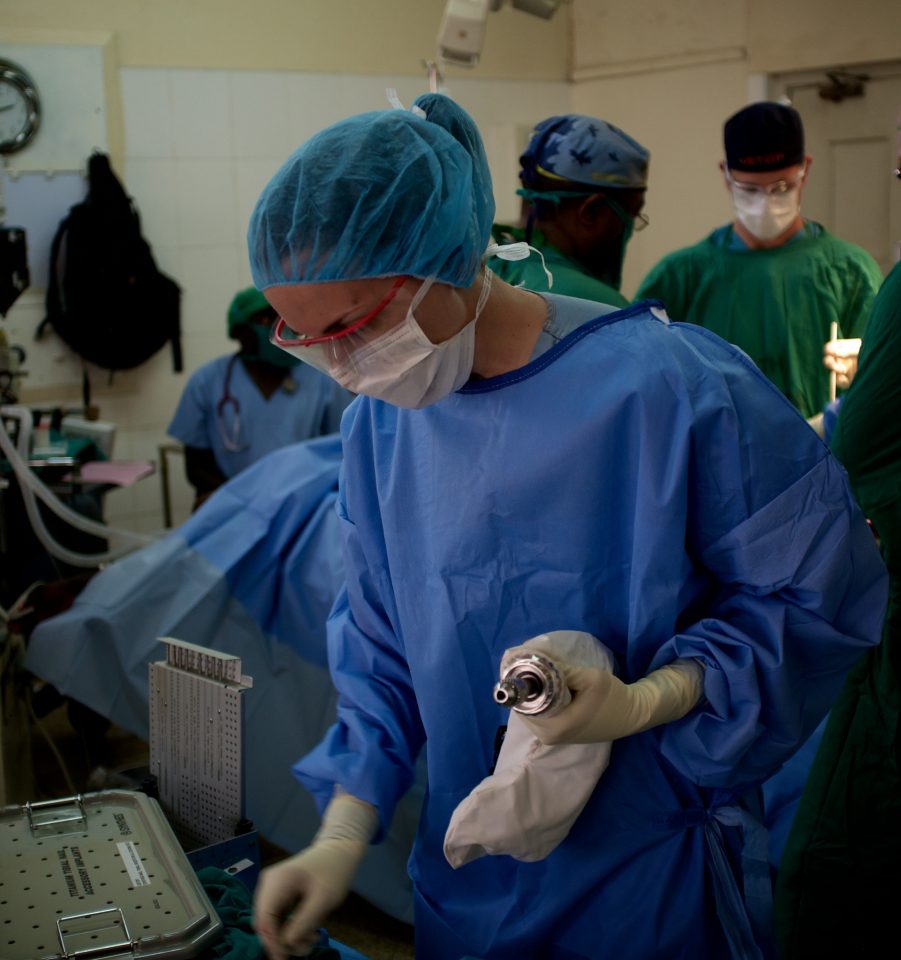
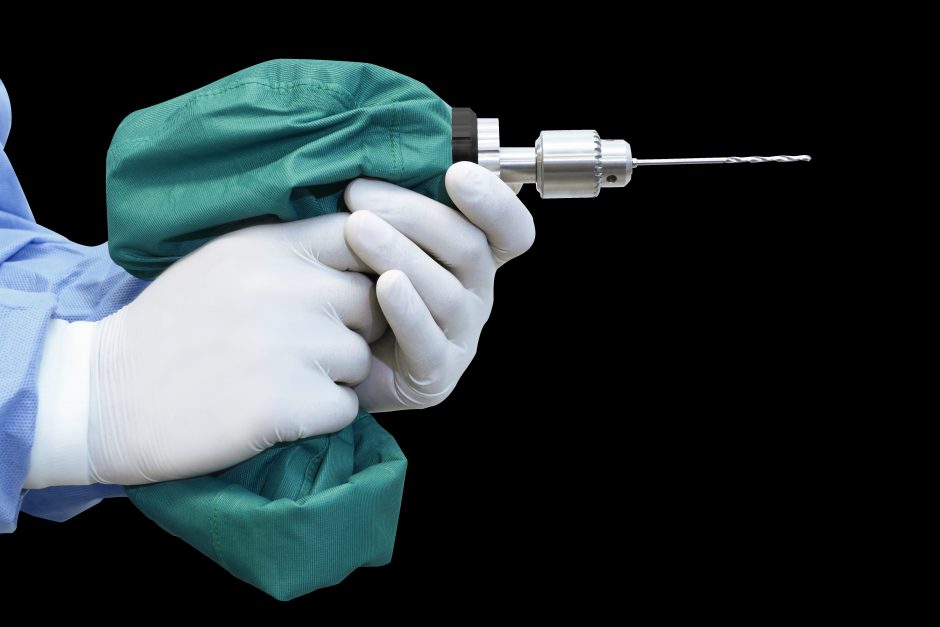
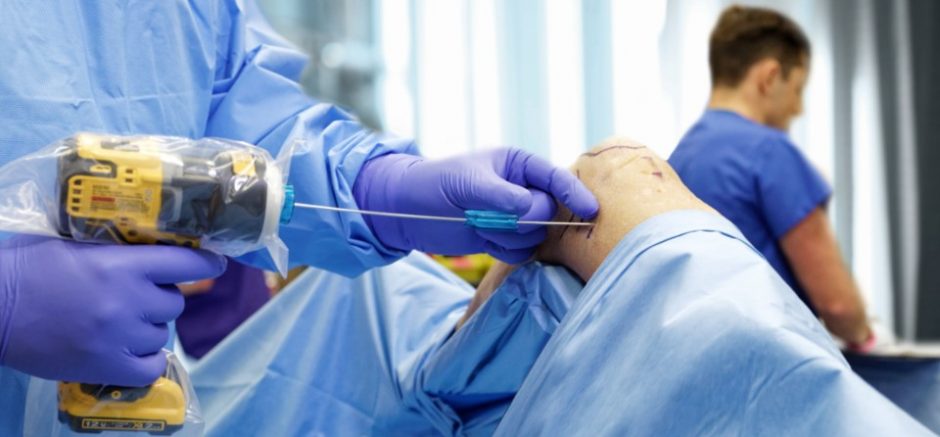

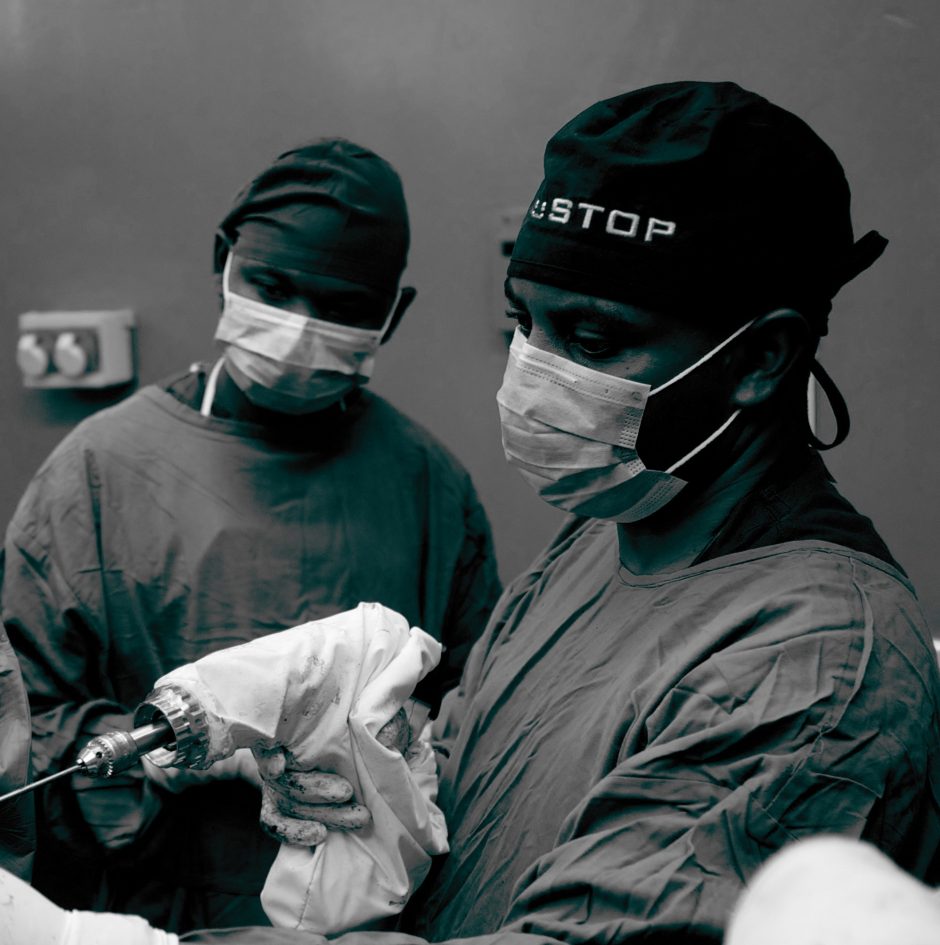

About Arbutus Medical: Arbutus Medical Inc. is a privately held ISO13485 medical device company headquartered in Vancouver, Canada. Arbutus Medical is developing innovative surgical drill technology and sterile-packed orthopaedic procedure kits that save time and money for hospitals. The company is currently focused on improving the standard of care for bedside skeletal traction surgeries in the ER of Trauma Centers through its TrakPak® product line. In addition to TrakPak®, the company distributes a line of surgical drills with DrillCover Technology that are available at accessible price points to customers around the globe. Arbutus Medical’s products have regulatory clearances from the U.S. Food & Drug Administration (FDA) and Health Canada, and have been used by customers in 40 countries, enabling an estimated 100,000 surgeries to date. The company’s mission is to simplify surgery, reduce costs, and help healthcare providers treat patients worldwide. For more information about Arbutus Medical, visit arbutusmedical.com and follow the company on LinkedIn.





